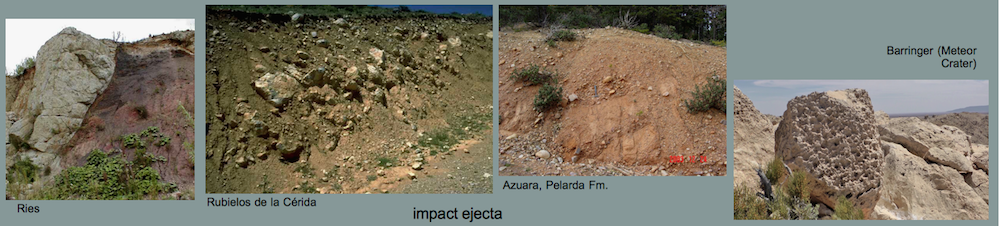
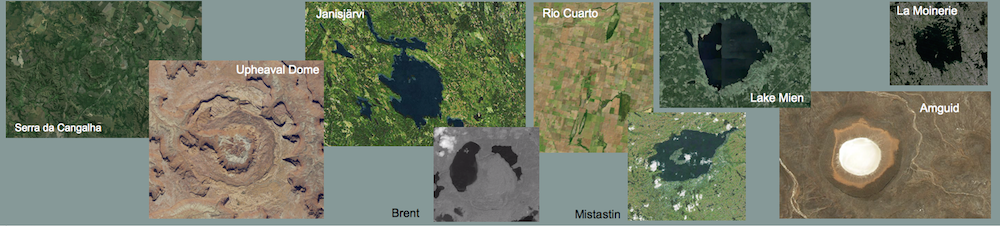
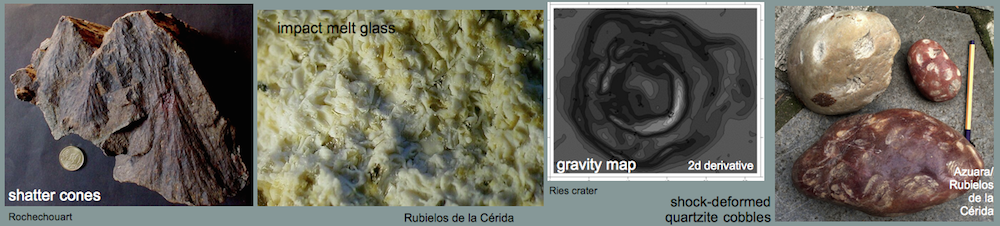
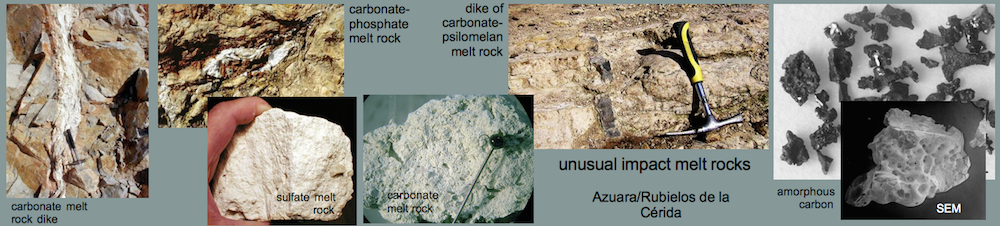
Impact melts from the Rubielos de la Cérida impact basin
Silicate melt
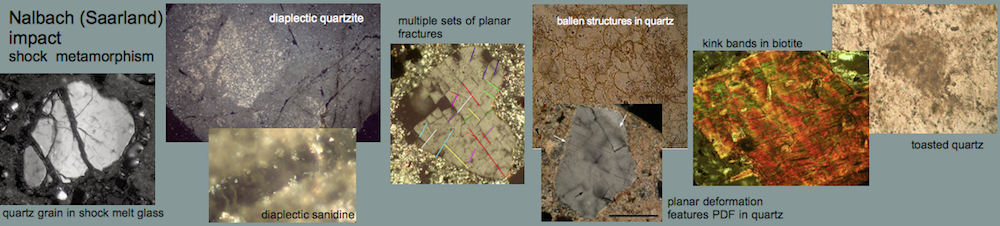
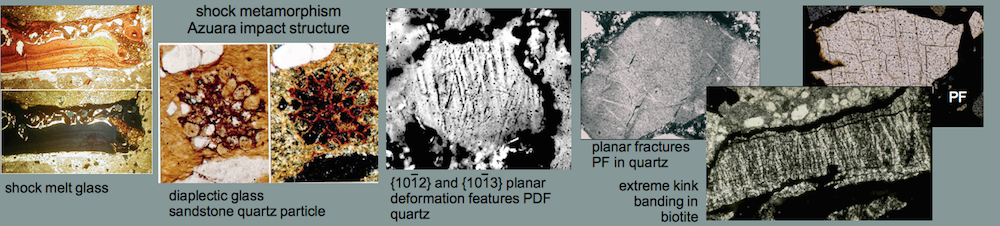
Melt rocks occur as soft, porous, fine-grained, whitish blocks of variable size in a range of decimeters up to 1 – 2 meters, intermixed in the polymictic Barrachina megabreccia. Two of these blocks have been investigated in detail (see images, x-ray powder diffractogram and table below). The rocks mainly consist of a milky white glass which forms tiny spheroids and lens-shaped bodies. Their diameters are roughly 0.5 mm. A second, subordinate glass phase is translucent grayish and occurs interstitial within the white glass particles. The glass is estimated to make up more than 90% of the rock. This is typically shown by a distinct amorphous glass “hump“, occurring in x-ray powder diffractograms. Some relics of plagioclase and, to a minor extent, of quartz and mica within the glass masses are indicated by respective reflection peaks. Grains of quartz, twinned plagioclase and occasional mica are also found in thin sections of the glass matrix. In rare cases, the quartz fragments show planar deformation features (PDFs) and, more frequently, multiple sets of planar fractures (PFs). Feldspar grains show isotropization in the form of multiple sets of isotropic twinning lamellae and isotropic spots (diaplectic crystals), and they have sometimes become almost completely isotropic (diaplectic glass), indicating shock peak pressures of the order of 30 GPa (300 kbar) (Engelhardt et al., 1969) (see Shock metamorphism).
Melt rocks occur as soft, porous, fine-grained, whitish blocks of variable size in a range of decimeters up to 1 – 2 meters, intermixed in the polymictic Barrachina megabreccia. Two of these blocks have been investigated in detail (see images, x-ray powder diffractogram and table below). The rocks mainly consist of a milky white glass which forms tiny spheroids and lens-shaped bodies. Their diameters are roughly 0.5 mm. A second, subordinate glass phase is translucent grayish and occurs interstitial within the white glass particles. The glass is estimated to make up more than 90% of the rock. This is typically shown by a distinct amorphous glass “hump“, occurring in x-ray powder diffractograms. Some relics of plagioclase and, to a minor extent, of quartz and mica within the glass masses are indicated by respective reflection peaks. Grains of quartz, twinned plagioclase and occasional mica are also found in thin sections of the glass matrix. In rare cases, the quartz fragments show planar deformation features (PDFs) and, more frequently, multiple sets of planar fractures (PFs). Feldspar grains show isotropization in the form of multiple sets of isotropic twinning lamellae and isotropic spots (diaplectic crystals), and they have sometimes become almost completely isotropic (diaplectic glass), indicating shock peak pressures of the order of 30 GPa (300 kbar) (Engelhardt et al., 1969) (see Shock metamorphism).
Four bulk samples of the melt rocks were analyzed by RFA Philips PW1480, and separated particles of the white and the grayish glass were measured using a CAMECA SX50 electron microprobe with wavelength dispersive spectrometers at operating conditions of 15 kV accelerating voltage, 15 nA beam current and a defocused beam size. The results are given in the Table below. Contents of Mn, Cr, Sc, Co, Ni, Mo and S are below the detection limit of the respective instrument. The poor totals of the microprobe analyses are most probably due to the presence of H2O which entered the glass by alteration. If corrected by LOI-determination, the totals are close to 100 % as shown for the bulk analyses.
The compositions of the milky white glass spheroids and the interstitial grayish glass particles do not differ significantly. The same holds true if microprobe analyses and RFA bulk analyses are compared. Major oxides are SiO2 between 53 and 59 wt.% and Al2O3 around 20 wt.%. The content of MgO in the glass particles (around 7 wt.%) is somewhat higher than in the bulk analyses (4.8 – 6.1 wt.%). Differences between the microprobe and the bulk analyses may be ascribed to secondary pore fillings or the mineral content.
 Fig. 1. Silicate melt rock (yellowish clast) in an outcrop of a multicolored breccia as part of the Barrachina megabreccia.
Fig. 1. Silicate melt rock (yellowish clast) in an outcrop of a multicolored breccia as part of the Barrachina megabreccia.
 Fig. 2. Patches of silicate melt in Lower Tertiary claystones. Barrachina megabreccia.
Fig. 2. Patches of silicate melt in Lower Tertiary claystones. Barrachina megabreccia.
 Fig. 3. Ribbon of silicate melt in the Barrachina megabreccia.
Fig. 3. Ribbon of silicate melt in the Barrachina megabreccia.
 Fig. 4. Silicate melt in contact with grayish Lower Tertiary claystone.
Fig. 4. Silicate melt in contact with grayish Lower Tertiary claystone.
 Fig. 5.The silicate melt rock under the optical microscope. The rock is composed of estimated more than 90 % glass forming tiny spheroids and lens-shaped bodies. The field is about 15 mm wide.
Fig. 5.The silicate melt rock under the optical microscope. The rock is composed of estimated more than 90 % glass forming tiny spheroids and lens-shaped bodies. The field is about 15 mm wide.
 Fig. 6. The silicate melt rock under the SEM. 100 µm scale bar.
Fig. 6. The silicate melt rock under the SEM. 100 µm scale bar.
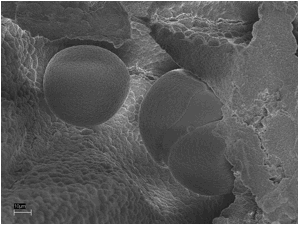 Fig. 7. The silicate melt rock under the SEM. 10 µm scale bar.
Fig. 7. The silicate melt rock under the SEM. 10 µm scale bar.
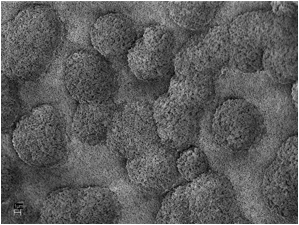 Fig. 8. The silicate melt rock under the SEM. 1 µm scale bar. SEM Images: ZEISS.
Fig. 8. The silicate melt rock under the SEM. 1 µm scale bar. SEM Images: ZEISS.
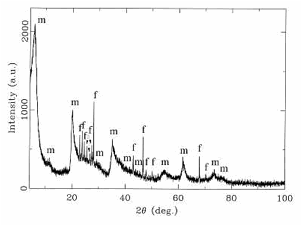 Fig. 9. X-ray powder diffractogram of the silicate melt rock. Beside the sharp diffraction peaks belonging to the feldspar phase (f), strongly broadened mica peaks (m) can be observed. The broadening reflects the low crystallinity of this phase. In the 2 (Theta)-range between 20° and 30°, a typical glass “hump” is displayed, with peaks of feldspar and mica phases superimposed.
Fig. 9. X-ray powder diffractogram of the silicate melt rock. Beside the sharp diffraction peaks belonging to the feldspar phase (f), strongly broadened mica peaks (m) can be observed. The broadening reflects the low crystallinity of this phase. In the 2 (Theta)-range between 20° and 30°, a typical glass “hump” is displayed, with peaks of feldspar and mica phases superimposed.
| wt.% | white | white | white | white | white | white | mean | wt.% | bulk-1 | bulk-2 | bulk-3 | bulk-4 | bulk-5 | ||
| SiO2 | 59,95 | 59,72 | 59,38 | 57,19 | 59,95 | 59,18 | 59,23 | SiO2 | 56,06 | 58,13 | 53,45 | 54,47 | 19,78 | ||
| TiO2 | 0,24 | 0,24 | 0,21 | 0,20 | 0,23 | 0,20 | 0,22 | TiO2 | 0,33 | 0,34 | 0,38 | 0,45 | 0,24 | ||
| Al2O3 | 20,75 | 19,53 | 19,88 | 21,30 | 23,16 | 18,63 | 20,54 | Al2O3 | 20,91 | 19,76 | 20,40 | 20,96 | 6,34 | ||
| MgO | 7,26 | 7,49 | 7,42 | 6,14 | 6,45 | 8,21 | 7,16 | MgO | 5,81 | 4,77 | 5,24 | 6,14 | 12,62 | ||
| CaO | 0,88 | 1,04 | 0,92 | 0,99 | 1,09 | 1,17 | 1,02 | CaO | 1,48 | 1,56 | 1,72 | 0,98 | 22,56 | ||
| FeO | 1,61 | 1,77 | 1,62 | 1,89 | 1,85 | 1,73 | 1,75 | FeO | 2,00 | 2,70 | 2,76 | 2,49 | 2,68 | ||
| Na2O | 1,92 | 1,87 | 1,82 | 1,63 | 1,56 | 1,66 | 1,74 | Na2O | 0,48 | 1,20 | 0,29 | 0,48 | 0,02 | ||
| K2O | 0,23 | 0,28 | 0,27 | 0,21 | 0,18 | 0,26 | 0,24 | K2O | 0,65 | 1,34 | 0,45 | 0,57 | 1,82 | ||
| Total | 92,84 | 91,94 | 91,52 | 89,55 | 94,47 | 91,04 | 91,89 | LOI | 10,30 | 9,24 | 14,02 | 11,70 | 32,91 | ||
| Total | 98,02 | 99,04 | 98,71 | 98,24 | 98,97 | ||||||||||
| wt.% | grey | grey | grey | grey | grey | mean | ppm | ||||||||
| SiO2 | 56,45 | 56,89 | 58,05 | 59,54 | 57,12 | 57,61 | V | 14 | 21 | 27 | 23 | ||||
| TiO2 | 0,27 | 0,21 | 0,26 | 0,22 | 0,25 | 0,24 | Zn | 36 | 46 | 68 | 81 | ||||
| Al2O3 | 20,81 | 19,88 | 19,66 | 15,99 | 22,74 | 19,82 | Ga | 35 | 38 | 30 | 33 | ||||
| MgO | 6,77 | 6,34 | 7,18 | 6,90 | 5,93 | 6,62 | Rb | 16 | 38 | 5 | 7 | ||||
| CaO | 1,14 | 1,17 | 1,23 | 1,24 | 1,14 | 1,18 | Sr | 492 | 363 | 327 | 364 | ||||
| FeO | 1,68 | 2,18 | 1,63 | 1,51 | 1,79 | 1,76 | Y | 43 | 37 | 32 | 38 | ||||
| Na2O | 1,42 | 1,19 | 1,49 | 0,79 | 1,31 | 1,24 | Zr | 493 | 475 | 491 | 522 | ||||
| K2O | 0,21 | 0,28 | 0,24 | 0,23 | 0,19 | 0,23 | Nb | 56 | 50 | 47 | 53 | ||||
| Total | 88,75 | 88,14 | 89,74 | 86,42 | 90,47 | 88,70 | Ba | 1250 | 171 | 48 | 1034 | ||||
| Pb | 79 | 238 | 29 | 31 | |||||||||||
| Th | 68 | 59 | 64 | 59 |
Table 1. Electron microprobe analyses of white and grey glass particles separated from the silicate glass, and mean compositions. X-ray fluorescence bulk analyses of four samples of the silicate glass (bulk-1 to bulk-4) and one melt-containing inclusion of the suevite (bulk-5).
Opponents of the impact (geologists from the Zaragoza university, e.g.. A. L. Cortés, and the Center of Astrobiology, Madrid, e.g., E. Díaz-Martínez [now at the Geological Survey of Spain], and others) insist on a volcanic origin (volcanic ash) of the silicate melt, although they never presented any analysis. We wonder why also Dr. M. R. Rampino from the New York university has considered the Barrachina silicate melt to be volcanic ash (written communication) although he, too, never supplied any explanation not to mention any analysis.
The Rubielos de la Cérida silicate glass rocks are clearly not of volcanic origin, due to the occurrence of strongly shocked clasts in the melt. Moreover, if these melt rocks were to represent a deformed ash layer, the rocks should contain pyroclastic fragments and, with respect to an “intermediate“ SiO2-concentration, mafic relic minerals or andesitic rock fragments. Such is clearly not the case. Moreover, the chemical composition should be similar to that of andesites or basaltic andesites. Those rocks, however, generally have distinctly lower contents of Al2O3 and much higher contents of FeO, CaO and (Na2O+K2O) than the investigated silicate melt rocks (a comparison was carried out with all analyses of volcanic rocks given in Wilson [1989]). Furthermore, the melting temperature estimated for the investigated rocks does not really match the temperatures in an andesite volcanic system.
Carbonate-phosphate melt
Carbonate-phosphate meltA very special kind of former melt was found within the Barrachina megabreccia. The whitish melt rocks (see Figs. 10-13) are composed of irregular spheroids up to 4 mm in size, which are embedded within an extremely fine-grained matrix. Under the microscope, the spheroids turn out to be globular to amoeba-like calcite particles. They are coarse-grained in their centers and display decreasing grain size towards the rims. Regularly, a perpendicular grain orientation towards the rims is observed. The contact with the matrix is extremely fine-grained (see photomicrograph below). The isotropic glass matrix in part is intensively pervaded by tiny, elongated, sometimes flaser-like microcrystals, often orientated tangentially to the rim of the calcite particles. The whole rock composition yields 52.7 wt.% CaO, 8.3 wt.% P2O5, and 1.5 wt.% BaO (RFA, bulk in Table below). From microprobe investigations, the carbonate of the particles is pure calcite. The glassy matrix mainly consists of CaO and P2O5 (Table below), with minor contents of F (1.0-2.5 wt.%), S (1.1-2.1 wt.%, if calculated as SO3), Cl (0.5-0.8 wt.%) and NaO (0.3-0.6 wt.%). The poor totals of the analyses point to high amounts of light components within the Ca-P-glass, presumably H2O which may have entered the glass during corrosion. The existence of considerable amounts of C or CO2, however, must also be taken into account. Locally, a strong enrichment of Ba and S at the expense of the CaO and P2O5 –content is observed, which is lowered to the range of trace elements or below the detection limit, whereas Al2O3 is present in minor concentrations of about 1 wt.%. In part, the Ca-P-glass is recrystallized to form apatite, as verified by x-ray powder diffraction analysis. The diffraction peaks of this apatite, however, are broadened compared to those of a well crystallized one (not shown here), indicating its very low crystallinity (see diagram below). The existence of baryte has also been proved by x-ray diffraction analysis. This baryte may occur as a very fine-grained phase within the Ba- and S-enriched locations in the Ca-P-matrix, detected by microprobe analysis.
 Fig. 10. Clast of carbonate-phosphate melt rock (white) in the Barrachina megabreccia. Coin diameter 23 mm.
Fig. 10. Clast of carbonate-phosphate melt rock (white) in the Barrachina megabreccia. Coin diameter 23 mm.
 Fig. 11. Carbonate-phosphate melt: surface of a break.
Fig. 11. Carbonate-phosphate melt: surface of a break.
 Fig. 12. Carbonate-phosphate melt in close-up: Calcite bodies (darker) in a matrix of phosphate glass (white).
Fig. 12. Carbonate-phosphate melt in close-up: Calcite bodies (darker) in a matrix of phosphate glass (white).
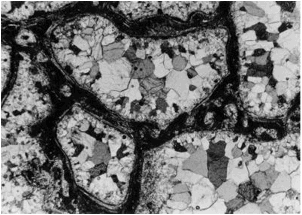 Fig. 13. Carbonate-phosphate melt rock: Photomicrograph (crossed polarizers) ofamoebae-like calcite bodies within a matrix of phosphate glass (dark). Note that the size of the individual calcite crystals increases towards the centers of the bodies. Also note that the peripheral calcite obviously has grown perpendicular to the rim because of the orientation. In part, especially along the borders to the calcite bodies, the phosphate glass has recrystallized to form apatite (elongated, sometimes flaser-like minerals tangentially orientated to the calcite bodies). The field is 6 mm wide.
Fig. 13. Carbonate-phosphate melt rock: Photomicrograph (crossed polarizers) ofamoebae-like calcite bodies within a matrix of phosphate glass (dark). Note that the size of the individual calcite crystals increases towards the centers of the bodies. Also note that the peripheral calcite obviously has grown perpendicular to the rim because of the orientation. In part, especially along the borders to the calcite bodies, the phosphate glass has recrystallized to form apatite (elongated, sometimes flaser-like minerals tangentially orientated to the calcite bodies). The field is 6 mm wide.
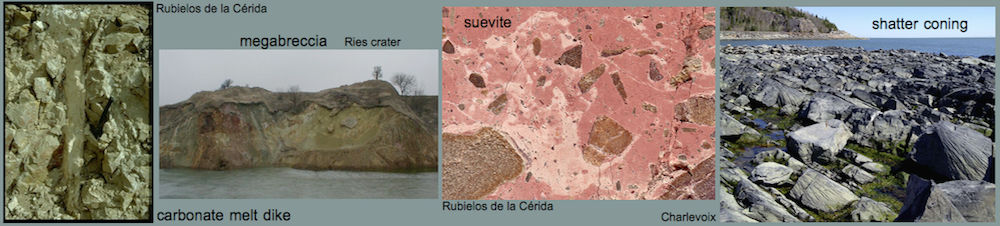
A similar melt has been reported for the suevite of the Ries crater. In the suevite, the calcite particles have identical structure and composition (Fig. 14) compared with the melt rocks of Barrachina (Fig. 13) and are interpreted by Graup (1999) as quench products of a carbonate melt. Different from the Barrachina melt rocks, the matrix in the Ries samples is silicate glass as a result of carbonate-silicate liquid immiscibility. In our case, the melt rock displays a small-scaled immiscibility of coexisting former carbonate melt and phosphate melt.
 Fig. 14. Carbonate-silicate liquid immiscibility in the Ries crater suevite (Graup 1999). Photomicrograph, crossed polarizers. The field is 5 mm wide.
Fig. 14. Carbonate-silicate liquid immiscibility in the Ries crater suevite (Graup 1999). Photomicrograph, crossed polarizers. The field is 5 mm wide.
| wt.% | 1 | 2 | 3 | 4 | 5 | 6 | mean | bulk | |
| P2O5 | 22,13 | 21,26 | 24,47 | 27,52 | 32,61 | 32,42 | 26,74 | 8,25 | |
| Al2O3 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | |
| CaO | 35,83 | 35,97 | 37,46 | 42,93 | 48,76 | 51,62 | 42,10 | 52,65 | |
| BaO | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 1,47 | |
| Na2O | 0,35 | 0,32 | 0,46 | 0,57 | 0,53 | 0,55 | 0,46 | 0,23 | |
| SO3 | 1,67 | 1,15 | 1,77 | 2,12 | 1,47 | 1,37 | 1,59 | 0,92 | |
| F | 1,57 | 1,56 | 1,02 | 2,26 | 2,24 | 2,39 | 1,84 | n.d. | |
| Cl | 0,62 | 0,71 | 0,50 | 0,79 | 0,49 | 0,55 | 0,61 | n.d. | |
| LOI | 34,31 | ||||||||
| Total | 62,18 | 60,98 | 65,68 | 76,19 | 86,10 | 88,91 | 73,34 | 97,83 | |
Table 1. Electron microprobe analyses of the glassy phosphate matrix, mean composition of this matrix, and x-ray fluorescence measured bulk composition of the carbonate-phosphate melt.
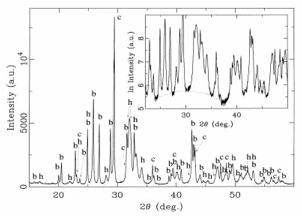 Fig. 15. X-ray powder diffractogram of the carbonate-phosphate melt. Peaks of calcite (c) and baryte (b) and distinctly broadened peaks of hydroxylapatite (h) are superimposed on a glass “hump”. The “hump” shows in the enlarged part of the diffractogram with a logarithmic scale for the intensity, but is less distinct due to a subordinate content of phosphate glass in the melt.
Fig. 15. X-ray powder diffractogram of the carbonate-phosphate melt. Peaks of calcite (c) and baryte (b) and distinctly broadened peaks of hydroxylapatite (h) are superimposed on a glass “hump”. The “hump” shows in the enlarged part of the diffractogram with a logarithmic scale for the intensity, but is less distinct due to a subordinate content of phosphate glass in the melt.
Carbonate melt rock
As already suggested for the Azuara structure, abundant relics of former carbonate melt are proposed to also occur in the Rubielos de la Cérida impact basin. A carbonate melt cannot be chilled to form glass, but rapidly crystallizes to carbonate again (see, e.g., the discussion by Graup, G. (1999). MAPS, 34, 425-438). Therefore, the origin from a melt can only indirectly be suggested by the occurrence of skeletal, dendritic crystallites, vesicular texture and related features (see, e.g., the discussion on Azuara carbonate melts by Katschorek [1990]).
 Fig. 16. Presumably carbonate melt rock from the Corbalán limestone quarry, southern impact basin. Close-up below.
Fig. 16. Presumably carbonate melt rock from the Corbalán limestone quarry, southern impact basin. Close-up below.
 Fig. 17. The low-density, highly porous material shows a distinct vesicular texture (the field is 7 mm wide).
Fig. 17. The low-density, highly porous material shows a distinct vesicular texture (the field is 7 mm wide).
 Fig. 18. Coating and cotton wool-like scraps of white carbonate material in a strongly vesicular calcitic framework: possibly relics of carbonate melt. Megabreccia between Escorihuela and El Pobo/Corbalán; southeastern rim of the impact basin.
Fig. 18. Coating and cotton wool-like scraps of white carbonate material in a strongly vesicular calcitic framework: possibly relics of carbonate melt. Megabreccia between Escorihuela and El Pobo/Corbalán; southeastern rim of the impact basin.
 Fig. 19. Close-up.
Fig. 19. Close-up.
 Fig. 20-22. SEM images of the relics of carbonate melt. Note the vesicular felted texture (this image) and the needle-shaped (Fig. 21) and the dendritic (Fig. 22) crystallites. SEM analyses establish calcium carbonate and traces of quartz. 20 µm scale bar.
Fig. 20-22. SEM images of the relics of carbonate melt. Note the vesicular felted texture (this image) and the needle-shaped (Fig. 21) and the dendritic (Fig. 22) crystallites. SEM analyses establish calcium carbonate and traces of quartz. 20 µm scale bar.
 Fig. 21. Needle-shaped calcium carbonate crystallites. 10 µm scale bar.
Fig. 21. Needle-shaped calcium carbonate crystallites. 10 µm scale bar.
 Fig. 22. Dendritic calcium carbonate crystallites. 2 µm scale bar.
Fig. 22. Dendritic calcium carbonate crystallites. 2 µm scale bar.
Sulfate melt rock
In the Barrachina megabreccia, white clasts (see Fig. 23) are embedded that consist of highly porous material (dry-rock densities of only 1.4 g/cm³ were measured). Only a few rock fragments are interspersed (Fig. 24). Chemically, the white material is nearly pure CaSO4. In thin section, the matrix may show flow texture but is otherwise not resolved microscopically. Mineral fragments, mostly quartz and feldspar, are partly strongly shocked (PDFs, diaplectic glass). Shock effects occur also in minerals of the interspersed rock fragments.
Evidently, the CaSO4 material is not a chemical sediment (gypsum, anhydrite), and a pedogenic origin can likewise be excluded. With respect to the high porosity, the flow texture and the strong shock effects, we suggest the clasts to have formed by crystallization from a shock-produced sulfate melt. The melting point of anhydrite is 1,450°C, a temperature which must have clearly been exceeded to produce the silicate melt in the Barrachina megabreccia. Crystallization from a sulfate melt is also discussed for material from the Haughton Dome impact structure (Osinski and Spray 2003): http://adsabs.harvard.edu/abs/2003E&PSL.215..357O
 Fig. 23. Clast of presumably sulfate melt rock in the Barrachina megabreccia. Coin for scale.
Fig. 23. Clast of presumably sulfate melt rock in the Barrachina megabreccia. Coin for scale.
 Fig. 24. The sulfate melt rock in close up. Note the quartzite clasts in the low-density, highly porous CaSO4 matrix.
Fig. 24. The sulfate melt rock in close up. Note the quartzite clasts in the low-density, highly porous CaSO4 matrix.
 Fig. 25. The sulfate melt rock under the SEM. Note the vesicular texture.
Fig. 25. The sulfate melt rock under the SEM. Note the vesicular texture.
Impact glass from shock, or pseudotachylite
The glass to be discussed here is coating a sandstone exposed in the southern part of the central uplift chain of the Rubielos de la Cérida impact basin. The glass has a greenish to whitish color and is transparent to milky. In thin section, the sandstone shows heavily damaged, and intense cataclastic flow texture is observed to merge with the glass. Quartz grains are strongly fractured and may show multiple sets of planar fractures (PFs) and planar features (probably PDFs).
Because of the shock effects in the sandstone, a shock origin of the peculiar glass seems reasonable. As an additional possibility, we suggest frictional melting by extreme dynamic metamorphism in the impact event (excavation or – more probably – modification stage when the uplift formed) and the glass to be pseudotachylite.
 Fig. 26. Shock-produced or pseudotachylite? Glass coating a sandstone in the southern uplift chain.
Fig. 26. Shock-produced or pseudotachylite? Glass coating a sandstone in the southern uplift chain.
 Fig. 27. The glass in close-up.
Fig. 27. The glass in close-up.
 Fig. 28. The glass-bearing sandstone cut perpendicularly to the glass crust (in the upper part). The field is 16 cm wide.
Fig. 28. The glass-bearing sandstone cut perpendicularly to the glass crust (in the upper part). The field is 16 cm wide.
 Fig. 29 The sawed surface in close-up: Note the complex flow field and injected (?) dikelets.
Fig. 29 The sawed surface in close-up: Note the complex flow field and injected (?) dikelets.
 Fig. 30. Photomicrograph (xx nicols; the field is 6 mm wide) of the glass-bearing sandstone below the glass crust (upper part).
Fig. 30. Photomicrograph (xx nicols; the field is 6 mm wide) of the glass-bearing sandstone below the glass crust (upper part).
 Fig. 31. Photomicrograph (xx nicols; the field is 240 µm wide) of the glass-bearing sandstone; three sets of planar features in a quartz grain.
Fig. 31. Photomicrograph (xx nicols; the field is 240 µm wide) of the glass-bearing sandstone; three sets of planar features in a quartz grain.